Background
SARS-CoV-2 was first identified after sequencing relevant clinical samples in a bunch of unknown viral pneumonia cases in December 2019 in Wuhan, Hubei Province, China. COVID-19, caused by SARS-CoV-2, was subsequently declared a pandemic by the WHO due to its aggressive spread on a large scale in many countries, leading to thousands of confirmed cases worldwide every day. As of 15 November 2020, 53.7 million confirmed cases of COVID-19 and 1.3 million deaths have been reported worldwide, demanding an urgent need for early identification of severe cases.1 Clinical evidence of SARS-CoV-2 has suggested several transmission routes between humans, with respiratory aerosol droplets undoubtedly being the main source of infection. SARS-CoV-2 is able to attack the respiratory system by binding to the cell entry receptors ACE2 on airway epithelial cells and results in pneumonia and respiratory failure in critically ill patients.
Chronic bronchitis, chronic obstructive pulmonary disease (COPD) and asthma are common respiratory diseases with chronic airway inflammation.2–4 Eosinophils, neutrophils and macrophages in innate immune response significantly increase in the airway and lungs during the initial phase of inflammation. Lymphocytopaenia has been reported in severe patients infected with SARS-CoV-2.5 Circulating eosinophil counts have also been reported to be decreased in patients with COVID-19 and associated with severity of the disease.6 Therefore, patients with underlying COPD, asthma and chronic bronchitis may have different inflammatory states after SARS-CoV-2 infection compared with patients without chronic airway inflammation.
In this retrospective cohort study, we reviewed the medical records of 59 patients with laboratory-confirmed COVID-19 with underlying chronic airway inflammation and compared the demographic, clinical and radiological characteristics as well as laboratory results between severe and non-severe patients in this cohort. Potential predictors of disease severity were identified in the abnormal laboratory findings using univariate and multivariate regression models.
Methods
Study population and data collection
The subjects of this study were adults with COVID-19 and underlying chronic respiratory diseases (admission date from 26 January to 3 April 2020) at the Sino-French New City Branch of Tongji Hospital. Severe and non-severe patients were included in the case and control groups, respectively. COVID-19 was diagnosed according to WHO interim guideline.7 Patients with chronic respiratory diseases were diagnosed according to a previous diagnosis. All patients were confirmed by positive findings in reverse-transcriptase PCR assay of SARS-CoV-2 RNA in throat swab specimens. The study was conducted on 15 June.Demographic information, clinical characteristics (including medical history, symptoms, comorbidities, smoking history and allergic history) and radiological results of each patient were obtained from the electronic medical record system of the Sino-French New City Branch of Tongji Hospital and analysed by three independent researchers. Severity of COVID-19 was staged according to the guidelines for diagnosis and treatment of COVID-19 published by the Chinese National Health Committee (version 5–7).
Criteria for severity of COVID-19
Severe COVID-19 was diagnosed when patients met one of the following criteria: (1) respiratory distress with respiratory frequency ≥30 per minute; (2) pulse oximeter oxygen saturation ≤93% at rest; and (3) oxygenation index (artery partial pressure of oxygen/inspired oxygen fraction) ≤300 mm Hg.
Laboratory testing
Medical laboratory results, including number of leucocytes, lymphocytes, monocytes, eosinophils, basophils, platelets, alanine aminotransferase, aspartate aminotransferase, serum creatine kinase, serum lactate dehydrogenase (LDH), blood urea nitrogen, serum creatinine, cardiac troponin I, concentrations of D-dimer, C reactive protein (CRP), procalcitonin, erythrocyte sedimentation rate, serum ferritin, cytokines (interleukin (IL) 2R, IL-6, IL-8, IL-10, tumour necrosis factor (TNF)-α) and immune function were collected for each patient from the electronic medical records.
Statistical analysis
All data were analysed with SPSS Statistics Software (V.26). The statistics for categorical variables were summarised as frequencies and percentages and were compared using χ2 test or Fisher’s exact test between different groups where appropriate. Continuous variables were described using median (IQR) and compared using Mann-Whitney U test. To explore the risk factors associated with disease severity, univariable and multivariable logistic regression models were used to estimate the OR and 95% CI. A two-sided α of less than 0.05 was considered statistically significant.
Results
Demographics and clinical characteristics of patients with non-severe and severe COVID-19 with chronic airway diseases
A total of 1888 patients were admitted. Fifty-nine patients with underlying chronic airway inflammation, including COPD (0.95%), asthma (0.53%) and chronic bronchitis (1.64%), were confirmed to have SARS-CoV-2 infection. Of the patients, 33 were classified as non-severe and 26 were classified as severe. Although COPD was more common in patients with severe COVID-19 when compared with patients with non-severe COVID-19 (42% vs 21%), the difference was not statistically significant.
The median age of all patients was 71 years (IQR, 57–80) and more than half (54%) were over 70 years old. Majority (71%) of the patients were male (table 1). There was no significant difference in age and sex between non-severe and severe patients. Thirty-one (53%) patients had one or more comorbidities besides the three chronic airway diseases, with cardiovascular disease (46%) and endocrine system disease (15%) being the most common comorbidity. There were no significant differences in the presence of these comorbidities between patients with non-severe and severe COVID-19. Half of the patients had smoking histories or were current smokers.
Demographics and clinical characteristics of patients with COVID-19 with chronic airway inflammation on admission
The most common symptoms were fever (83%), cough (73%), fatigue (47%) and dyspnoea (42%). Dyspnoea was more common in severe patients compared with non-severe patients (65% vs 24%, p=0.001) (table 1).
Laboratory findings of patients with non-severe and severe COVID-19 with chronic airway diseases
When compared with non-severe patients, severe patients were more likely to have elevated neutrophil counts (8.2×10⁹/L vs 4.1×10⁹/L, p=0.001), decreased lymphocyte counts (0.6×10⁹/L vs 1.1×10⁹/L, p<0.001), eosinopaenia (<0.02×10⁹/L; 73% vs 24%, p<0.001), elevated D-dimer (>1 µg/mL; 88% vs 42%, p=0.001), increased LDH (471.0 U/L vs 230.0 U/L, p<0.001), elevated blood urea nitrogen (>9.5 mmol/L; 42% vs 3%, p<0.001), increased hypersensitive troponin I (>34 pg/mL; 48% vs 7%, p=0.001), and increased inflammation markers including CRP (126.2 mg/L vs 19.9 mg/L, p<0.001), procalcitonin (≥0.05 ng/mL; 96% vs 43%, p<0.001) and ferritin (1264.2 mg/L vs 293.6 mg/L, p=0.004) (table 2). Of note, significant differences in the expression of inflammation-related cytokines including IL-6, IL-8 and TNF-α were observed between the two groups, which were dramatically increased in severe patients.
Laboratory findings of patients with COVID-19 with chronic airway inflammation on admission
Predictors of severity of COVID-19 in patients with chronic airway diseases
To identify the predictors of severity of COVID-19 in patients with chronic airway diseases, we analysed the association between abnormal laboratory findings and disease severity with univariate and multivariate logistic regression models. Disease severity was significantly associated with all of the above-mentioned abnormal laboratory findings in univariate logistic regression analyses. In a multivariate regression model that incorporated lymphopaenia, eosinopaenia, elevated LDH and increased IL-6, eosinophil counts <0.02×10⁹/L (OR per one-unit decrease, 10.115 (95% CI 2.158 to 47.414), p=0.003) and LDH level >225 U/L (OR per one-unit increase, 22.300 (95% CI 2.179 to 228.247), p=0.009) were independent risk factors for disease severity (table 3). Our data suggest that decreased eosinophil counts and increased LDH levels may help clinicians identify severe COVID-19 in patients with chronic airway diseases.
Predictors of severity of COVID-19 in patients with chronic airway diseases
Eosinophil counts and LDH levels tend to return to normal range over time in non-severe patients
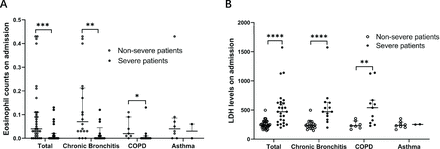
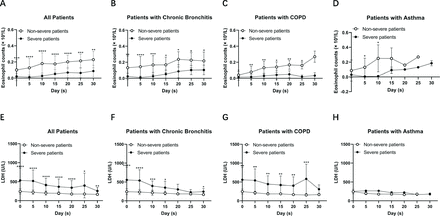
We further analysed the eosinophil counts and LDH levels in patients with non-severe and severe COVID-19 with chronic bronchitis, COPD and asthma, respectively. We found that there was a significant difference in eosinophil counts and LDH levels between severe and non-severe patients with chronic bronchitis and COPD, but not in patients with asthma (figure 1). To observe the dynamic changes of eosinophil counts and LDH levels over time, we collected the eosinophil counts and LDH levels on the 5th, 10th, 15th, 20th, 25th and 30th days after admission. We found that eosinophil counts increased over time both in severe and non-severe patients. Meanwhile, LDH decreased over time (figure 2). Severe patients showed a slower recovery rate than non-severe patients. Of note, both eosinophil counts and LDH levels recovered more slowly in severe patients with COPD than those in severe patients with chronic bronchitis. Our data suggest that, as the disease recovers, eosinophil counts and LDH levels tend to return to normal range both in severe and non-severe patients, indicating a good therapeutic effect in patients with chronic airway diseases in COVID-19 treatment.
Clinical characteristics of eosinophil and LDH in patients with COVID-19 with chronic airway inflammation. (A) Eosinophil counts in different subgroups. Eosinophil counts were significantly decreased in patients with severe COVID-19 with chronic bronchitis and COPD. (B) LDH levels in different subgroups. LDH levels were significantly decreased in patients with severe COVID-19 with chronic bronchitis and COPD. Values for non-severe and severe patients are presented with open and closed circles, respectively. Mann-Whitney U test was used. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. COPD, chronic obstructive pulmonary disease; LDH, lactate dehydrogenase.
Dynamic changes of eosinophil counts and LDH levels in patients with COVID-19 with chronic airway diseases. (A–D) Eosinophil counts increased over time in non-severe and severe patients with COVID-19 with chronic bronchitis (n=31), COPD (n=18) and asthma (n=10). (E–H) LDH levels decreased over time in non-severe and severe patients with COVID-19 with chronic bronchitis (n=31), COPD (n=18) and asthma (n=10). Values for non-severe and severe patients are presented with open and closed circles, respectively. Mann-Whitney U test was used. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, compared with the eosinophil counts or LDH levels between severe and non-severe patients. COPD, chronic obstructive pulmonary disease; LDH, lactate dehydrogenase.
We further performed multivariate analysis for mortality in patients with COVID-19 with chronic airway inflammation using the above four variables and found that eosinophil count <0.02×10⁹/L (OR per one-unit decrease, 18.000 (95% CI 1.929 to 167.986), p=0.011) was the only independent risk factor for mortality (online supplemental table 1). Moreover, Kaplan-Meier survival curves indicated that patients with COVID-19 with eosinopaenia or elevated LDH had worse survival probability (p<0.05) (online supplemental figure 1). This suggests that eosinopaenia and elevated LDH are also potential predictors of mortality in patients with COVID-19 with underlying chronic airway diseases.
Supplemental material
Discussion
In this retrospective cohort study, we found that eosinophil counts less than 0.02×10⁹/L and LDH levels greater than 225 U/L on admission were associated with severity of COVID-19 in patients with underlying chronic bronchitis, COPD and asthma. Moreover, eosinophil counts and LDH levels tend to return to normal range in severe and non-severe patients after treatment, suggesting their roles as indicators of disease progression and treatment efficacy.
Circulating and tissue-resident eosinophils are associated with a variety of diseases, in which eosinophils participate in the pathological process and play a potent proinflammatory role, such as COPD, asthma and chronic bronchitis. In view of elevated eosinophils in patients with chronic airway inflammation, COPD, asthma and chronic bronchitis have not yet been reported as major risk factors for severity of SARS-CoV-2 infections. Zhang et al
8 reported that none had asthma or other comorbid atopic diseases and only two patients had COPD (1.4%) in a cohort of 140 hospitalised patients with COVID-19, more than half of whom (53%) had eosinopaenia on the day of hospital admission. Similarly, Du et al
9 analysed the clinical features of 85 fatal cases of COVID-19 and found that 81% of the patients had very low eosinophil counts on admission. In our cohort including 1888 patients, 31 patients had chronic bronchitis (1.64%), 18 patients had COPD (0.95%) and only 10 patients had asthma (0.53%). Meanwhile, eosinopaenia was more common in critically severe patients, suggesting that the resolution of eosinopaenia could be a possible way to improve clinical status.10 In our study, lower count of eosinophils showed worse survival probability, and eosinophil counts significantly decreased in patients with severe COVID-19 with chronic bronchitis and COPD. No significant difference was observed in patients with asthma, partly due to the limited sample size. Moreover, drastically increased eosinophil counts in the airways of most patients with chronic asthma after bronchoprovocation might be another more important cause. We further explored the dynamic changes of eosinophil counts in patients with chronic airway diseases in the course of COVID-19 and found that eosinophil counts gradually increased over time and returned to normal range in both severe and non-severe patients, which could be a possible indicator of treatment effectiveness. It remains unclear how eosinopaenia takes place in COVID-19, but the most possible reason could be due to its depletion of antiviral reaction, since Th1 (Type 1 T helper) antiviral response was inhibited in those patients with chronic airway inflammation.
LDH has long been reported to be associated with COPD, asthma and chronic bronchitis and identified as a potential marker of chronic airway inflammation.11 12 Meanwhile, a large number of studies reported elevated LDH levels in COVID-19, which could be a risk factor for mortality. Zheng et al
13 conducted a systematic literature review and meta-analysis including four studies and found that LDH was statistically significantly higher in severe patients compared with non-severe patients. Elevated LDH in severe cases indicated diffuse lung injury and tissue damage; therefore, we hypothesised that LDH might be another predictor of chronic airway inflammation exacerbation in COVID-19. Kaplan-Meier survival analysis suggested the hazard of elevated LDH levels. Similar to eosinophil, LDH showed elevated levels in patients with severe COVID-19 with chronic bronchitis and COPD and gradually decreased over time in patients with severe and non-severe COVID-19.
Multiple research has highlighted the important roles of eosinopaenia and elevated LDH in facilitating the diagnosis and prognosis of severe COVID-19. Ma et al
14 included eosinopaenia to introduce the COVID-19-REAL (radiological image, eosinophils, age, and leukocytes) score, which had a good performance in identifying populations at higher risk of getting COVID-19. Cazzaniga et al
15 reported that absolute eosinopaenia in the binary logistic regression analyses was associated with 4-week mortality and clinical outcomes in patients with COVID-19 pneumonia.15 Guan et al
16 and Feng et al
17 both proposed LDH as a significant predictor of COVID-19 mortality and adverse outcomes with the simple-tree XGBoost model, which may help identify high-risk COVID-19 cases. Eosinopaenia and elevated LDH have been identified as risk factors for severe COVID-19 cases; however, it is noteworthy that in our study they were also associated with severity of COVID-19 in patients with chronic airway diseases.
There is growing concern regarding the association between COVID-19 and pulmonary function. Previous reports have concentrated on respiratory follow-up after hospitalisation for COVID-19. Trinkmann et al
18 found that symptomatic patients had a significantly lower forced expiratory volume in 1 s, vital capacity and transfer factor of the lung for carbon monoxide (TLCO) compared with asymptomatic patients. Riou et al
19 found that patients with critical disease had lower total lung capacity and TLCO. However, how the pre-existing pulmonary function impairment impacted the COVID-19 outcome has not yet been fully elucidated. Currently, related literature is scarce, possibly due to limited pulmonary function data and the temporary closure of pulmonary function testing laboratories during the COVID-19 pandemic. Morgenthau et al
20 found that mortality in patients with COVID-19 with sarcoidosis was associated with moderate or severe impairment in pulmonary function. He et al
21 reported that a longer history of COPD increased the risk of death and negative outcomes of patients with COVID-19, which was consistent with He’s work.21 In our cohort, the impaired lung function of patients with COVID-19 with underlying chronic airway diseases might have a significant impact on the outcome. However, the analysis could not be conducted due to unavailable data, which was a limitation of this study.
Previous treatment regimens might contribute to the outcome of patients with COVID-19 with underlying chronic airway diseases. Inhaled corticosteroids (ICS) (with or without long-acting β-agonist) are applied directly to the respiratory epithelium in the intervention of stable COPD and asthma to reduce airway inflammation. ICS could decrease the expression of both ACE2 and transmembrane protease serine 2 (TMPRSS2) on airway epithelial cells, subsequently protecting them from being invaded by SARS-CoV-2.22 In addition, the proliferation of coronavirus and cytokine production could also be suppressed by the usage of ICS.23 However, whether the use of regular ICS before the pandemic had an impact on COVID-19 outcomes remains controversial. Bloom et al reported that patients with asthma older than 50 years could benefit from the use of ICS within 2 weeks of admission, while patients with other chronic pulmonary diseases could not.24 Schultze et al
25’s work denied the positive role of regular ICS use in protecting against severe outcome of COVID-19, both in patients with asthma and in patients with COPD. In our cohort, only one patient with COPD and two patients with asthma reported having long-term use of ICS due to the difficulty in collecting medical histories in the initiation of the pandemic. Further detailed information on comorbidities, prior medication and many bias factors should be taken into account to figure out the benefits or harms of ICS in COVID-19.
Different phenotypes of COPD and asthma based on the complex pathophysiology might also be partly involved in COVID-19; however, the hypotheses need to be further clarified. Kimura et al
26 found that type 2 inflammatory cytokines (IL-4, IL-5, IL-13) were negatively associated with ACE2 expression while positively associated with TMPRSS2 expression in an ex vivo study. Ferastraoaru et al
27’s work indicated that a Th2 asthma phenotype was a predictor of reduced COVID-19 morbidity and mortality, while Kermani et al
28 reported greater morbidity and mortality outcome in neutrophilic severe asthma. A previous report has highlighted that eosinophilic inflammation was also a common and stable phenotype in COPD and blood eosinophil counts could predict response to ICS treatment.29 Watson et al did not find any gene expression differences in ACE2 in blood eosinophilic COPD, further indicating that these patients might not have a different vulnerability to SARS-CoV-2 infection.30 Therefore, how different inflammation types of COPD and asthma might impact the progress of severe COVID-19 needs further investigation.
Our study also had some other limitations. First, due to the retrospective study design, the accuracy of all laboratory results was dependent on medical records. Observation bias might also exist in this study due to the limited sample size. Second, there could be a selection bias in the multivariate regression model when analysing the risk factors.